After your testing is completed, your provider will go over the results with you. At that time you can decide which advanced treatment options would be best for you.

Available advanced treatment options for treatment of refractory overactive bladder.
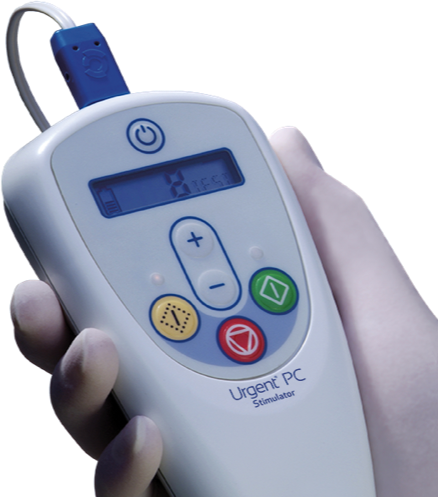
Urgent® PC
Made by Laborie
cogentixmedical.com
Urgent® PC is a non-drug, non-surgical option for overactive bladder and symptoms of urinary urgency, frequency and urge incontinence.
Indications
PTNS is indicated for patients with overactive bladder who have tried conservative therapy and medications without significant improvement. Patients who have adverse, intolerable, side effects from medications are also good candidates for PTNS.
How it Works
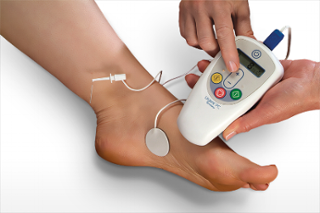
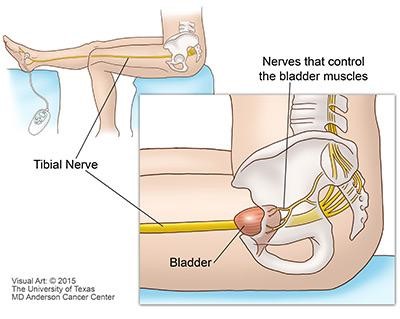
Neuromodulation therapy is the name given to a group of treatments that deliver electrical pulses to nerves to change how they work. One of these therapies is PTNS. PTNS modifies the signals the bladder receives and processes in order to achieve improved bladder control. Using an electrode (small needle) inserted in the inner ankle electrical impulses are sent up the tibial nerve to “calm down” the signals coming from the sacral nerves which control your bladder. The nerves in the sacral plexus will stop “firing” so often which can decrease symptoms of urgency and frequency.
Expected Results
- Due to the way that PTNS works, it usually takes 5-7 weeks to see a change in symptoms. Patients report different time courses with improvement. No identifiable traits have been identified to determine who will respond early, late or respond at all to treatment. Therefore, it is important to receive all 12 recommended treatments to determine how well PTNS works. If successful, maintenance therapy likely will be necessary to maintain the improvement.
- Most studies report an improvement in incontinence (typical reduction 1-3 episodes/day), frequency (reductions of 2-5 episodes/day), nocturia (reductions of 1-2 episodes/night) and quality of life.
- Studies show that, on average, over 60% of patients have an improvement.
- PTNS works as well as antimuscarinic medications with a better safety profile.
Safety
Who should NOT be treated with PTNS?
- Patients with pacemakers or implantable defibrillators
- Patients prone to excessive bleeding
- Patients with nerve damage that could impact the tibial nerve or pelvic floor
- Pregnant patients or patients who may become pregnant during therapy
Common side effects
- Temporary mild pain, bleeding or skin inflammation at or near the stimulation site.
BOTOX®(Onabotulinumtoxin A)
Made by Allergan
www.botoxforoab.com
Indications
Botox is a prescription medicine injected into the bladder muscle. Indicated to treat OAB symptoms and refractory urge incontinence in patients when another type of medication does not work well enough or is not tolerated. It is also indicated for leakage due to OAB in patients with neurologic disease who still leak or cannot tolerate the side effects of medication.
How it Works
Small doses of this drug can paralyze muscles. When injected into the bladder muscle, this drug may help keep it from contracting too often. BOTOX works by blocking the nerve signals in your bladder that cause the unstable, overactive bladder contractions. Over time, this treatment wears off and it may need to be repeated in 6 months or a year.
Procedure
BOTOX is injected in multiple areas of your bladder muscle using a cystoscope (Small lighted camera inserted through the urethra) and a special needle which allows us to inject the medication at the exact depth we want. This can be performed in the clinic or in the operating room. The procedure takes approximately 30 minutes and upon discharge, minimal adverse effects are noted.
Depending on whether your procedure is done under local anesthetic or in the operating room with an anesthesiologist will determine whether you can leave immediately and whether you will be able to drive yourself home after the injection of BOTOX.
After the procedure, you will be able to leave in a very short time and should not have any significant discomfort or interference with your normal routine. You should be able to get back to normal activities the following day.
Expected Results
BOTOX can provide up to 6 months of improvement in symptoms in patients who have not been able to tolerate anticholinergic medications or had incomplete success with medications.
Results are not immediate as it may take several weeks to see a change in urinary symptoms.
Symptom improvement (leakage episodes) varies but approximately 2 out of 3 patients experience more than 50% improvement, one out of two patients achieve more than 75% improvement and approximately 30% of patients improve 100%.
The improvement in symptoms includes decreasing daily leakage, improving urgency and decreasing urinary frequency.
Overall quality of life improvement is not just based on decreasing urinary symptoms, but also on improvements in avoidance behaviors (bathroom mapping, fluid restriction), frustration and embarrassment. In most trials, the measured quality of life was significantly improved.
Common Side Effects
- Urinary tract infection (18%)
- Dysuria (9%) is painful or uncomfortable urination
- Urinary retention (6%): Difficulty emptying your bladder is a very frustrating side effect. If you are unable to empty your bladder you may need to perform intermittent catheterization
There are important safety considerations when utilizing BOTOX which are important to understand both for the patient and the provider.
Do not use BOTOX if you have an active urinary tract infection or are not able or willing to initiate catheterization after the injection procedure. Due to the risk of urinary retention only patients who are willing and able to do intermittent catheterization should be considered for treatment.
Do not take BOTOX® if you: are allergic to any of the ingredients in BOTOX® ; had an allergic reaction to any other botulinum toxin product such as Myobloc® Dysport®, or Xeomin®; have an infection at the planned injection site.
Serious side effects can occur and if you experience any of these please get medical attention right away. These problems have been reported to occur at any time (hours to weeks) after injection.
Problems swallowing, speaking, or breathing, due to weakening of associated muscles, can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms including loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing.
BOTOX® may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours to weeks of taking BOTOX®. If this happens, do not drive a car, operate machinery, or do other dangerous activities.
Serious and/or immediate allergic reactions have been reported. They include itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint.
Get medical help right away if you experience symptoms; further injection of BOTOX® should be discontinued.
Prior to treatment tell your doctor:
- If you have significant neurologic conditions such as ALS or Lou Gehrig’s disease, myasthenia gravis, or Lambert-Eaton syndrome, as you may be at increased risk of serious side effects including difficulty swallowing and difficulty breathing
- If you have autonomic dysreflexia from a spinal cord injury as this can increase blood pressure to unsafe levels
- If you have a bleeding disorder which might increase risks of the injection procedure
- If you have a urinary tract infection as it may increase your risk for a more severe infection
- If you are pregnant or plan on becoming pregnant. Unknown risk for unborn baby
- If you are breastfeeding as it is unknown if botox passes into the breast milk
- If you have received any other botulinum toxin product in the last 4 months; have received injections of botulinum toxin such as Myobloc®, Dysport®, or Xeomin® in the past
InterStim™
Made by Medtronic
www.medtronic.com
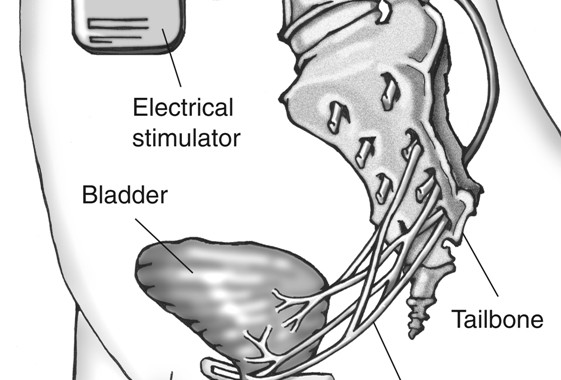
Sacral neuromodulation is a reversible therapy used to treat overactive bladder, urinary incontinence, and incomplete bladder emptying. As of 2018, the InterStim device is the only FDA approved sacral neuromodulation device in the US, but several others are in development. The InterStim device consists of a small battery that is implanted under the skin in your buttock, which sends mild electrical pulses via a wire that sits under the tailbone, near the sacral nerves. These nerves control the bladder and the muscles related to urinary function. The pulses sent by the InterStim device help smooth out overactive connections between the bladder to the brain, which can lead to an improvement in overactive bladder symptoms.
Indications
InterStim therapy is used for patients with urge incontinence, urgency, and frequency who have failed other treatment options. It is reserved for patients who have not had adequate improvement in behavioral therapy or medications. It is a third line option along with PTNS and BOTOX.
In addition, Interstim can be used for urinary retention and fecal incontinence. Patients who have both urinary and fecal incontinence are often encouraged to consider InterStim, as it may improve both problems. More information regarding this can be found on Medtronic’s website. Click here for a link.
How it Works
A small battery (about the size of a pacemaker) is be placed under your skin. This delivers mild electrical pulses to the nerves that control your bladder. This therapy works by stimulating the sacral nerve (near the tailbone). This nerve carries signals between the brain and the bladder. In overactive bladder, these nerve impulses can occur too frequently. Sacral neuromodulation uses a “bladder pacemaker” to smooth out these overactive signals, which can improve OAB symptoms.
Procedure
Placement of the InterStim device is a 2 stage process. The first step is a minimally invasive test either done in the office or ambulatory center under local anesthesia in which involves placing one or more leads through the skin of your lower back to a location under your tailbone. These connect to an external battery pack that is either taped to your skin or worn on your clothing by clipping it to your waistband. You return to see your doctor four to fourteen days later to discuss how well it worked. You and your doctor can then determine if moving on to a permanent implant would be beneficial.
If the testing phase is successful, a permanent InterStim implant can be placed. This procedure involves finalizing the lead placement and implanting a small permanent battery under the skin.
Results
- 84.8% improvement in urgency urinary incontinence.
- 39% of patients need revision of the sacral neuromodulation implant
Al-zahrani AA1, Elzayat EA, Gajewski JB. Long-term outcome and surgical interventions after sacral neuromodulation implant for lower urinary tract symptoms: 14-year experience at 1 center. J Urol. 2011 Mar;185(3):981-6
Common Risks
- Pain at the stimulation site (15.3%)
- New pain (9%)
- Pain at the lead site (5.4%)
- Suspected lead migration (8.4%)
- Infection (6%)
- Transient electric shock (5.5%)
- Changes in bowel function (3%) have all been reported complications of a permanent implantation after a PNE testing
Siegel SW, Catanzaro F, Dijkema HE, et al. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology. 2000;56:87–91
- Medtronic performed a multicenter clinical study including 23 centers worldwide. A total of 581 patients were studied with 219 of them receiving InterStim Therapy. They defined success as increased voided volume with the same or reduced degree of urgency.
- Here are the results:
- Patients had a baseline degree of > 7 voids per day.
- Urge Incontinence (12 month results)
- 45% of patients remained completely dry
- 34% of patients had > 50% reduction in number of wetting episodes
- 70% of patients eliminated heavy leaking episodes
- Urgency-Frequency (12 month results):
- 33% of patients reduced the number of voids by 50%
- 31% of patients reduced the number to normal (4-7 per day)
- 82% of patients improved degree of urgency before a void
Source: Medtronic.com
